Electrolysis Of Water Lab Answers . Electrolysis of water is the process of decomposing water into. The electrolysis of water produces hydrogen and oxygen gases. — how to electrolyse water. let us understand first what is the electrolysis of water. The solution turns blue at the cathode (basic) and red at the anode (acidic). study with quizlet and memorize flashcards containing terms like water molecules, electrolysis, electrolyte and more. Two carbon pencil “leads” (graphite rods) will be inserted into the opposite ends of a petri. Twice as much gas is evolved at the cathode as at the anode. in this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like water) to. lysis of water in an electrochemical cell. The electrolytic cell consists of a pair of. Using a small power source and some electrodes, you can separate molecules of water into hydrogen and.
from askfilo.com
— how to electrolyse water. lysis of water in an electrochemical cell. let us understand first what is the electrolysis of water. Electrolysis of water is the process of decomposing water into. Twice as much gas is evolved at the cathode as at the anode. Two carbon pencil “leads” (graphite rods) will be inserted into the opposite ends of a petri. in this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like water) to. The electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of. study with quizlet and memorize flashcards containing terms like water molecules, electrolysis, electrolyte and more.
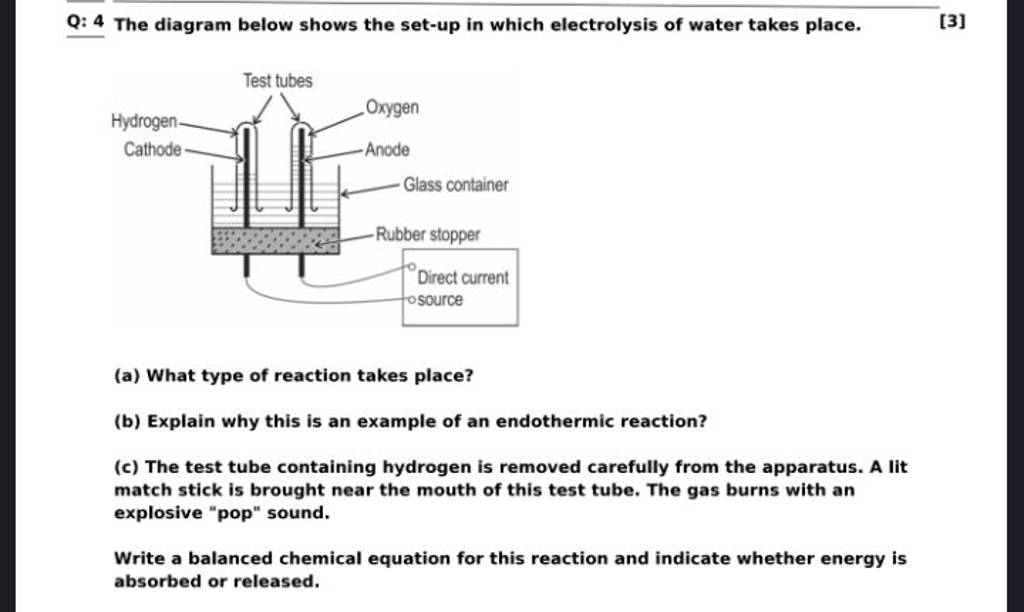
Q 4 The diagram below shows the setup in which electrolysis of water ta..
Electrolysis Of Water Lab Answers Using a small power source and some electrodes, you can separate molecules of water into hydrogen and. The electrolysis of water produces hydrogen and oxygen gases. let us understand first what is the electrolysis of water. Electrolysis of water is the process of decomposing water into. Two carbon pencil “leads” (graphite rods) will be inserted into the opposite ends of a petri. — how to electrolyse water. study with quizlet and memorize flashcards containing terms like water molecules, electrolysis, electrolyte and more. The electrolytic cell consists of a pair of. The solution turns blue at the cathode (basic) and red at the anode (acidic). Twice as much gas is evolved at the cathode as at the anode. in this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like water) to. Using a small power source and some electrodes, you can separate molecules of water into hydrogen and. lysis of water in an electrochemical cell.
From dokumen.tips
(PDF) Electrochemistry Electrolysis of Water Lab Weeblylpscience Electrolysis Of Water Lab Answers — how to electrolyse water. lysis of water in an electrochemical cell. study with quizlet and memorize flashcards containing terms like water molecules, electrolysis, electrolyte and more. Two carbon pencil “leads” (graphite rods) will be inserted into the opposite ends of a petri. Twice as much gas is evolved at the cathode as at the anode. The. Electrolysis Of Water Lab Answers.
From www.sciencephoto.com
Electrolysis of water Stock Image A500/0247 Science Photo Library Electrolysis Of Water Lab Answers Twice as much gas is evolved at the cathode as at the anode. Two carbon pencil “leads” (graphite rods) will be inserted into the opposite ends of a petri. The electrolytic cell consists of a pair of. The electrolysis of water produces hydrogen and oxygen gases. — how to electrolyse water. Using a small power source and some electrodes,. Electrolysis Of Water Lab Answers.
From www.numerade.com
SOLVED Observation of electrolysis of water before, during, and after Electrolysis Of Water Lab Answers The solution turns blue at the cathode (basic) and red at the anode (acidic). The electrolytic cell consists of a pair of. Electrolysis of water is the process of decomposing water into. The electrolysis of water produces hydrogen and oxygen gases. Using a small power source and some electrodes, you can separate molecules of water into hydrogen and. study. Electrolysis Of Water Lab Answers.
From chem.libretexts.org
17.7 A Deeper Look Electrolysis of Water and Aqueous Solutions Electrolysis Of Water Lab Answers The electrolytic cell consists of a pair of. let us understand first what is the electrolysis of water. The electrolysis of water produces hydrogen and oxygen gases. study with quizlet and memorize flashcards containing terms like water molecules, electrolysis, electrolyte and more. Electrolysis of water is the process of decomposing water into. Twice as much gas is evolved. Electrolysis Of Water Lab Answers.
From www.linkedin.com
Water Electrolysis Different Methods Electrolysis Of Water Lab Answers — how to electrolyse water. let us understand first what is the electrolysis of water. Twice as much gas is evolved at the cathode as at the anode. Using a small power source and some electrodes, you can separate molecules of water into hydrogen and. The solution turns blue at the cathode (basic) and red at the anode. Electrolysis Of Water Lab Answers.
From www.jargonium.com
Electrolysis and Education Learning Models not Facts Electrolysis Of Water Lab Answers let us understand first what is the electrolysis of water. Electrolysis of water is the process of decomposing water into. Two carbon pencil “leads” (graphite rods) will be inserted into the opposite ends of a petri. in this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like water) to. The. Electrolysis Of Water Lab Answers.
From slidetodoc.com
Electrolysis of Water Lab Report Electrolysis of Water Electrolysis Of Water Lab Answers lysis of water in an electrochemical cell. Using a small power source and some electrodes, you can separate molecules of water into hydrogen and. Twice as much gas is evolved at the cathode as at the anode. Electrolysis of water is the process of decomposing water into. The electrolysis of water produces hydrogen and oxygen gases. let us. Electrolysis Of Water Lab Answers.
From lessoncampusnuttiest.z22.web.core.windows.net
Experiment Electrolysis Of Water Electrolysis Of Water Lab Answers The electrolysis of water produces hydrogen and oxygen gases. — how to electrolyse water. Using a small power source and some electrodes, you can separate molecules of water into hydrogen and. in this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like water) to. study with quizlet and memorize. Electrolysis Of Water Lab Answers.
From lessonlibraryalluded.z13.web.core.windows.net
Electrolysis Of Water Experiment Lab Report Electrolysis Of Water Lab Answers Twice as much gas is evolved at the cathode as at the anode. study with quizlet and memorize flashcards containing terms like water molecules, electrolysis, electrolyte and more. let us understand first what is the electrolysis of water. in this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like. Electrolysis Of Water Lab Answers.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Electrolysis Of Water Lab Answers The solution turns blue at the cathode (basic) and red at the anode (acidic). let us understand first what is the electrolysis of water. The electrolysis of water produces hydrogen and oxygen gases. in this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like water) to. lysis of water. Electrolysis Of Water Lab Answers.
From askfilo.com
Q 4 The diagram below shows the setup in which electrolysis of water ta.. Electrolysis Of Water Lab Answers study with quizlet and memorize flashcards containing terms like water molecules, electrolysis, electrolyte and more. Electrolysis of water is the process of decomposing water into. The electrolysis of water produces hydrogen and oxygen gases. let us understand first what is the electrolysis of water. The solution turns blue at the cathode (basic) and red at the anode (acidic).. Electrolysis Of Water Lab Answers.
From kolblabs.com
Electrolysis of water in Chemical Reactions and Equations Class 10 Electrolysis Of Water Lab Answers — how to electrolyse water. study with quizlet and memorize flashcards containing terms like water molecules, electrolysis, electrolyte and more. Twice as much gas is evolved at the cathode as at the anode. Two carbon pencil “leads” (graphite rods) will be inserted into the opposite ends of a petri. in this lab you will use a battery. Electrolysis Of Water Lab Answers.
From www.studocu.com
Waterelectrolysis Super download don't download waste Electrolysis Of Water Lab Answers in this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like water) to. The electrolysis of water produces hydrogen and oxygen gases. lysis of water in an electrochemical cell. study with quizlet and memorize flashcards containing terms like water molecules, electrolysis, electrolyte and more. Two carbon pencil “leads” (graphite. Electrolysis Of Water Lab Answers.
From studylib.net
LAB Electrolysis of Water Electrolysis Of Water Lab Answers Twice as much gas is evolved at the cathode as at the anode. Electrolysis of water is the process of decomposing water into. study with quizlet and memorize flashcards containing terms like water molecules, electrolysis, electrolyte and more. Using a small power source and some electrodes, you can separate molecules of water into hydrogen and. lysis of water. Electrolysis Of Water Lab Answers.
From morrisclassicalacademy.blogspot.com
Morris Classical Academy Electrolysis of water Electrolysis Of Water Lab Answers — how to electrolyse water. Twice as much gas is evolved at the cathode as at the anode. study with quizlet and memorize flashcards containing terms like water molecules, electrolysis, electrolyte and more. The electrolytic cell consists of a pair of. Using a small power source and some electrodes, you can separate molecules of water into hydrogen and.. Electrolysis Of Water Lab Answers.
From studylib.net
THE ELECTROLYSIS OF WATER LAB ELEC.5 INTRODUCTION Electrolysis Of Water Lab Answers Two carbon pencil “leads” (graphite rods) will be inserted into the opposite ends of a petri. in this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like water) to. study with quizlet and memorize flashcards containing terms like water molecules, electrolysis, electrolyte and more. Electrolysis of water is the process. Electrolysis Of Water Lab Answers.
From edurev.in
Electrolysis of water is a reaction. The molar ratio of Electrolysis Of Water Lab Answers The electrolytic cell consists of a pair of. Twice as much gas is evolved at the cathode as at the anode. in this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like water) to. Using a small power source and some electrodes, you can separate molecules of water into hydrogen and.. Electrolysis Of Water Lab Answers.
From www.tessshebaylo.com
Water Electrolysis Chemical Equation Tessshebaylo Electrolysis Of Water Lab Answers The electrolysis of water produces hydrogen and oxygen gases. study with quizlet and memorize flashcards containing terms like water molecules, electrolysis, electrolyte and more. lysis of water in an electrochemical cell. let us understand first what is the electrolysis of water. The solution turns blue at the cathode (basic) and red at the anode (acidic). Twice as. Electrolysis Of Water Lab Answers.